- Innovative Medicine
- Neuroscience
Neuroscience
For nearly seven decades, we have pioneered innovative medicines that have significantly advanced treatment for nervous system disorders.
Despite significant advancements in understanding and treating nervous system disorders, unmet need persists for patients. That’s why, we’ll never stop pushing the boundaries of what is possible.
Disease areas
Innovation

Precision neuroscience
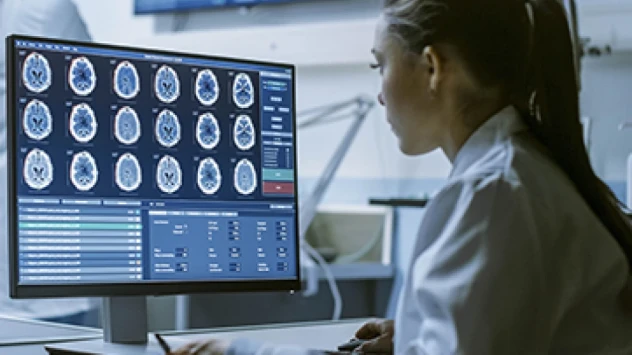
Next-generation measurement

Patient-centered outcomes
Learn more
Check out our pipeline
You are now leaving jnj.com/innovativemedicine
This link will take you to a different Johnson & Johnson website, where different laws and regulations may apply.
You are now leaving jnj.com/innovativemedicine
This link will take you to a different Johnson & Johnson website, where different laws and regulations may apply.
Our products
Depression looks like me
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.
Resources
Unseen and unheard
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.
Could we be on the cusp of catching Alzheimer’s before it starts?
Mental health journey: Greyson’s story
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.
It’s not just ink
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.
You are now leaving jnj.com
This link will take you to a website outside of Johnson & Johnson, which is governed by its own privacy policy and terms of use.













